Immune System SeriesB Cells and Antibodies
Each B cell is programmed to make one specific antibody. For example, one B cell will make an antibody that blocks a virus that causes the common cold, while another produces antibody that zeros in on a bacterium that causes pneumonia.
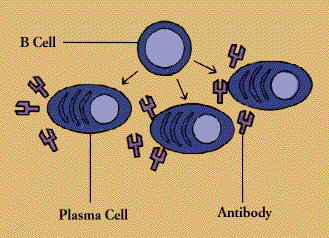
When a B cell encounters its triggering antigen(along with collaborating T cells and accessory cells), it gives rise to many large plasma cells. Every plasma cell is essentially a factory for producing antibody. Each of the plasma cells descended from a given B cell (which are all members of the same family, or clone) manufactures millions of identical antibody molecules and pours them into the bloodstream.
A given antibody matches an antigen much as a key matches a lock. The fit varies: sometimes it is very precise, while at other times it is little better than that of a skeleton key. To some degree, however, the antibody interlocks with the antigen and thereby marks it for destruction.
Antibodies belong to a family of large molecules known as immunoglobulins. Immunoglobulins are proteins, made up of chains of polypeptides, strings of the basic units known as amino acids. Each antibody has two identical heavy polypeptide chains and two identical light chains, shaped to form a Y. The sections that make up the tips of the Y's arms vary greatly from one antibody to another, creating a pocket uniquely shaped to enfold a specific antigen. This is called the variable (V) region. The stem of the Y serves to link the antibody to other participants in the immune defenses. This area is identical in all antibodies of the same class, and is called the constant (C) region.
Scientists have identified nine chemically distinct classes of human immunoglobulins (Ig)-four kinds of IgG and two kinds of IgA, plus IgM, IgE, and IgD. Each type plays a different role in the immune defense strategy. IgG, the major immunoglobulin in the blood, is also able to enter tissue spaces; it works efficiently to coat microorganisms, speeding their uptake by other cells in the immune system. IgM, which usually combines in star-shaped clusters, tends to remain in the bloodstream, where it is very effective in killing bacteria. IgA concentrates in body fluids-tears, saliva, the secretions of the respiratory and gastrointestinal tracts-guarding the entrances to the body. IgE, which under normal circumstances occurs only in trace amounts, probably evolved as a defense against parasites, but it is more familiar as the villain in allergic reactions (Allergy). IgD is almost exclusively found inserted into the membranes of B cells, where it somehow regulates the cell's activation.
Antibodies can work in several ways, depending on the nature of the antigen. Antibodies that interlock with toxins produced by certain bacteria can disable them directly (and are known as antitoxins). Other antibodies, by coating (or opsonizing) bacteria, make the microbes highly palatable to scavenger cells equipped to engulf and destroy them. More often an antigen-antibody combination unleashes a group of lethal serum enzymes known as complement (Complement). Yet other antibodies block viruses from entering into cells (a quality that is exploited in making vaccines). And, in a phenomenon known as antibody-dependent cell-mediated cytotoxicity (ADCC), cells coated with antibody become vulnerable to attack by several types of white blood cells.